Objective: The purpose of this study is to deepen the description of aggressive periodontitis in developmental age patients
going through pathogenesis, epidemiology, diagnosis, treatment and differential diagnosis.
Methods: The database searching was performed on PubMed and Scopus using the following keywords: “prepubertal
periodontitis, aggressive periodontitis, periodontitis in children, periodontal disease in children and adolescents, early-onset
periodontitis”. Both clinical, laboratorial and review studies were taken into consideration.
Results: Aggressive periodontitis affects a low percentage of
children, and in those patients. Actinomycetemcomitans is the
main bacterium identified in affected sites. Moreover, it has been found
that Genetics plays a fundamental role in development and
progression, which is important to distinguish the various oral
manifestations excluding the possibility that they are a consequence
of systemic pathologies. Although it mainly affects young patients, the
treatment does not differ from that applied in adult subjects
and it consists of a causal therapy, a mechanic and a pharmacological
one, in particular the antibiotics associated with professional
hygiene has shown very satisfactory results.
Discussion: Given the great variability of oral manifestation
symptoms, there is no specific criterion for defining a high-risk
group at prepubertal age, further research is needed to identify a
robust set of genetic, microbiological and host factors markers
that may facilitate the diagnosis of the disease.
Keywords: Aggressive periodontitis; periodontal disease; developmental age; differential diagnosis
Introduction
Periodontal disease is one of the most widespread diseases in
the world and is, as a prevalence, immediately after diseases such
as diabetes and hypertension [1]. Its clinical aspects have long been
analyzed since it constitutes a worldwide problem. The pathology
affects subjects of every race, sex and age and also some risk factors
are needed in addition to the individual susceptibility for it to
develop. Even today there are not enough scientific certainties to
establish the behavior of periodontal disease in the age group that
affects children and young adults. In any case, we proceeded through
a search in the literature with the attempt to deepen its clinical
aspects, etiology, diagnosis, predisposing factors and treatment.
Periodontal diseases, despite being widespread especially in the
adult population, are not so rare even among young people [2]. For
example, gingivitis affects over 70% of children over the age of seven
[3]. Bimstein in 1991 underlined the importance of prevention,
early diagnosis and treatment of periodontal diseases in children
and adolescents because they have a high severity and prevalence
[4] and furthermore, the oral-dental incipient pathologies on small
subjects can develop into periodontal diseases in adults. However,
the degree of extension and destruction of periodontitis also
responds to a personal predisposition to the disease. The severity
index of periodontal disease can also be mediated by the presence
of some systemic diseases such as hypophosphatasia or leukocyte
deposition deficiency [5].
According to Lamster IB and Pagan M, the metabolic syndrome
(MetS) that is a spectrum of conditions that include dysglycemia,
visceral obesity, atherogenic dyslipidemia (high triglycerides and low
levels of high-density lipoprotein) and hypertension are associated
with periodontal disease. They believe that this relationship is the
result of systemic oxidative stress and an exuberant inflammatory
response. Evidence suggests that periodontal therapy may reduce
serum levels of inflammatory mediators so periodontitis treatment
could become part of the metabolic syndrome therapy [6]. Among
the various types of periodontitis, one of the less studied ones
is aggressive periodontitis. The manifestations of aggressive
periodontitis in young people have many controversial sides
and consequently, the present study proposes to look for some
clarifications regarding the aspects of the pathology
Methods
For this narrative review, the database searching was
performed on PubMed and Scopus using the following keywords:
“prepubertal periodontitis, aggressive periodontitis, periodontitis
in children, periodontal disease in children and adolescents,
early-onset periodontitis”, the investigation then focused on
evaluating the specific aspects of aggressive periodontitis in
children, consequently the following words have been introduced:
“epidemiology, classification, progression, treatment, diagnosis”.
Clinical and laboratorial studies were taken into consideration as
well as literature reviews. The last database search was performed
in September 2019.
Discussion
Aggressive periodontitis
Aggressive periodontitis can occur in several forms that is
linked to a few dental sites or in a generalized sense. The first form
usually affects smaller subjects and is connected to lesions of the
first molars or incisors or both in the presence of little plaque and
tartar, and the second form concerns post-puberty subjects with
more permanent teeth. Very often, if left untreated, the localized
forms evolve into general forms with the risk of a total compromise
of the dental apparatus. Sometimes the signs of inflammation
are not so easily detectable, which is why a child or teenager on
the first visit should always be subjected to a more in-depth
analysis by using probes to detect probing depth and radiological
investigations. There are some mechanisms that regulate
evolution in the various age groups, and the different anatomies
and physiologies can modify the development of periodontitis. In
particular, there are many structural inequalities between adults
and children. The gingiva in the young is more vascularized, has
less connective tissue around the deciduous teeth, the epithelium
is thinner and less keratinized, characteristics that can expose to
less defense to attacks bacterial. It can be said that a child, due
to its thinness of tissues, is more exposed to risk and moreover a
greater vascularization allows an easier transit of inflammation
mediators and bacteria [7]. The typical signs that indicate the
presence of a problem and that should alarm the parents are
bleeding gums during home hygiene practices, swelling, halitosis
accompanied by any recessions. Evidence shows that periodontal
disease may increase during adolescence due to lack of motivation
to practice oral hygiene but also due to changes related to puberty.
Hormones such as progesterone, estrogen and testosterone cause
greater blood circulation, greater sensitivity and greater response
to any irritation, the gengiva are often red and swollen. Hormones
are molecules with specific regulatory abilities and have powerful
effects on the main determinants of development and on the
integrity of the skeletal cavity including periodontal tissues [8].
Epidemiology
A 1987 study by Sweeney [9] evaluated alveolar bone loss
around primary teeth in a population of 2,264 children. Nineteen
patients (0.84%) showed periodontal bone destruction around
one or more primary teeth; in 2 of these patients, periodontal
disease was previously identified during clinical examinations.
The microbiological study also revealed a high prevalence of
Actinobacillus actinomycetemcomitans and Capnocytophaga.
Another study carried out by Bimstein [10] in 1994 verified
the prevalence of alveolar bone loss in a group of 317 5-yearold
New Zealand children. The results identified that there was
a questionable bone compromise in 8.5% of the children and a
defined bone loss of 2.1%. Darby et al in 2005 studied bone loss in
542 children aged between 5 and 12 years. Reading the patient’s
radiographs, each interdental site was evaluated as: no bone loss
and therefore distance from the amelite-cementitious junction
to the alveolar ridge of less than 2 mm, questionable bone loss
i.e. distance greater than 2 mm but less than 3 mm and bone loss
defined or distance greater than or equal to 3 mm.
The results showed that 61 (13%) children presented sites
with definite bone loss, 60 children had only a questionable bone
loss, 50 children had only a defined bone loss and 21 children had
both lesions. It was also found that children of Asian-Far Eastern
origin had a higher percentage of sites with bone loss than children
of Caucasian origin, 29.5% and 19.7%, respectively, but lower than
that of children of Middle Eastern origin (35.2%). In conclusion, the
present study showed that in the population studied, 26% had bone
loss but 13% had more severe and defined lesions [11]. The studies
described above thus show that the prevalence of periodontal
disease and in particular bone loss varies from 0.84% to 13%,
but in reality, the heterogeneity of such research and the lack of
standardization makes it clear how the results are discordant and
the prevalence remains mostly dubious.
Microbiology
There are some bacteria that mainly cause periodontal disease,
and these can be transmitted within the family where the contact
between subjects is very close; through the mother’s saliva, for
example, children may be exposed to risk. Much attention has been
paid to Actinomycetemcomitans as a species implicated in the
etiology of aggressive periodontitis. Its main virulence factor is a
leukotoxin capable of eliminating important cells of the immune
system. Genetic analyses have identified a population structure of
the clonal-type bacterium with evolutionary families corresponding
to serotypes. A particular highly leukototoxic clone (JP2) of
serotype b was discovered. Its characteristics are unique in fact that
its increased leukototoxic activity is given by a deletion of 530
bases in the operon. The geographical mapping of the JP2 clone has
revealed that its colonization mainly concerns individuals of African
origin [12]. A study conducted by Burgess et al. in 2017 showed
the prevalence of the highly leukotoxic JP2 sequence compared to
the non-JP2 sequence of Aggregatibacter actinomycetemcomitans
within a group of 180 young African Americans aged between 5 and
25 years with and without localized aggressive periodontitis (LAP).
Subgingival plaque was collected from diseased sites, i.e. from
areas with probing depth greater than or equal to 5 mm that
presented bleeding and from healthy sites, i.e. from areas with
probing depth less than or equal to 3 mm that did not present
bleeding. Overall, 90 subjects (50%) tested positive for the JP2
sequence, 50 subjects (83.33%) with aggressive periodontitis
presented the sequence detected in 45 (75%) sick sites and 34
(56.67%) healthy sites [13]. Actinomycetemcomitans in general is
considered an opportunistic pathogen of the oral microbiome, in
fact many clonal types of the bacterium different from JP2 can be
isolated from healthy subjects, however, patients who present the
JP2 strain always show periodontal disease, so an etiological agent
is important for aggressive periodontitis in children, adolescents
and adults. In conclusion, there is a high risk for the development of
the disease in individuals colonized by the JP2 clone, furthermore
its transmission, as for other clonal types, occurs vertically by
close contact between people, indicating that subjects of the same
family may experience extrinsic routes of the subpopulation of the
bacterium [12].
Genetics
Genetics plays an important role in the appearance and
severity of the disease, so if a person is diagnosed with aggressive
periodontitis, it is also good to investigate the other members of
the family in order to cure or prevent their appearance. A 1994
study by Mary L Marazita studied evidence of autosomal dominant
inheritance and specific heterogeneity in aggressive periodontitis.
Analyses were conducted on 100 families, and 104 subjects were
diagnosed with aggressive periodontitis. Heterogeneity tests were
used to compare the parameter estimates and the conclusions
obtained in the black and non-black families. The results of the
segregation analysis have verified that an autosomal dominant
locus is sufficient to explain the patterns of disease transmission
to the whole family. In conclusion, in the present study, we saw
how the disease has a chance of appearing in the same branch of
descent at 70% [13]. Family aggregation of aggressive periodontitis
is not an unusual discovery. The conditions of development of
this pathology can be more complex than simple Mendelian
syndromes. Genetic studies indicate that there are several genetic
variants expressing different forms of aggressive periodontitis, but
currently it is not clear how many genes may be involved in these
non-syndromic forms of disease [14]. It is important to remember
that family models can also indicate exposure to common
environmental factors within the same family. Therefore, the
behavioral components shared by the same parental group must
also be considered: education, socioeconomic status, oral hygiene,
possible transmission of bacteria, diseases such as diabetes and environmental characteristics such as even passive smoking
influence the susceptibility of the subject as risk factors. Another
decisive reason for determining whether individuals develop
periodontitis appears to be regulated by the way they respond
to their microflora. Genetic factors also in this case modulate
the way in which individuals interact with many environmental
agents, including biofilm. The mutual influence of genetic and
environmental factors, and not only of genes, determines the result,
lifestyle factors open the way to the development of aggressive
disease [15].
Treatment
When a child is diagnosed with aggressive periodontitis, prompt
action must be taken to achieve maximum reduction of periodontal
microorganisms with the aim of blocking the development of a
more severe clinical picture. The treatment of aggressive disease
in children and adolescents does not differ from the techniques
applied to adults, in fact the etiology is always known to be bacterial
regardless of age group. The treatment therefore should be based
on the elimination of pathogens by professional hygiene, but, in
reality, in these forms of periodontal disease given the high toxicity
of the microorganisms, a systemic therapy with antibiotics must
be associated to resolve the picture. The goal is to create a clinical
condition that favors the maintenance of the greatest number of
teeth for as long as possible. The initial phase of active treatment
consists of mechanical cleaning, performed with or without the use
of antimicrobial drugs.
The downsizing and smoothing of the roots have proved
effective in improving the clinical indices, but they do not always
guarantee long-term stability, which is why systemic antibiotics as
adjuvants for radicular treatment are to be administered during
therapy, and they are more effective than root resizing alone with
the additional application of local or antiseptic antibiotics [16].
A 2005 study by Guerrero et al. evaluated the systemic
administration of amoxicillin and metronidazole in non-surgical
therapy for the treatment of generalized aggressive periodontitis.
Forty-one systemically healthy subjects in whom the disease
was diagnosed were selected. Patients received non-surgical
treatment over a 24-hour period and one half received a course
of systemic antibiotic consisting of 500 mg of amoxicillin and 500
mg of metronidazole three times a day for 7 days while the other
group of subjects received placebo. After two and six months, they
were re-evaluated and the results were as follows: in patients on
antibiotic therapy in the 7 mm pockets there was a gain of 1.4 mm
and a recovery of bone equal to 1 mm in addition to the areas with
depths greater than or equal to 5 mm had a probing less than or
equal to 4 mm. Twenty five percent of sites in test patients had a
successful improvement in clinical attack, whereas for patients
treated only with placebo, the percentage of improved sites was
16% [17]. Another study by Kaner shows how the subgingival
application of chlorhexidine via a controlled release device
(CHX chip) does not improve the clinical outcome in generalized
aggressive periodontitis. The purpose of that study is to compare
whether the additional positioning of the CHX chip is as effective as
the use of systemic antibiotics. A total of 36 patients were
diagnosed with aggressive periodontitis, one half was treated only
with slow-release chlorhexidine and the other half treated with
systemic antibiotic therapy. The subjects were re-evaluated 3 and
6 months after therapy, and it was shown that the level of clinical
attack, bleeding and probing depth had a significant improvement
in patients receiving amoxicillin and metronidaziol compared to
patients treated with the local application of antiseptics [18]. An
alternative method for the decontamination of periodontal sites
has been studied: photodynamic therapy. To reduce the excessive
use of antibiotics, new disinfection strategies have been sought.
Photodynamic therapy (PDT) or light-activated disinfection (LAD)
was first tested by Oscar Raab in the early 1900s.
For years it was abandoned due to the use of antibiotics, but it
has found a new application in the last decades both in the medical
field and in the dental field. The photodynamic reaction takes
advantage of the use of a photosensitizer (PS) and a light source
calibrated to specific wavelengths in the presence of oxygen. It
acts specifically against both Gram + and Gram- microorganisms
without causing any damage to the host cells. The toluidine blue is
very effective active against many bacteria including those involved
in periodontal disease. One example is Arweiler’s [19] research
in which the use of antibacterial photodynamic therapy (aPDT)
was studied in addition to mechanical scaling and root planning
therapy. The aim of that study was to evaluate the results following
non-surgical periodontal therapy and additional use of aPDT or
amoxicillin and metronidazole (AB) in patients with aggressive
periodontitis. Out of 36 patients treated with antibiotic therapy or
with two episodes of post-treatment photodynamic therapy, the
results after six months were the following: the probing depth was
found to be significantly reduced in both groups.
Despite this, the administration of amoxicillin and metronidazole
produced higher improvements than the existence of photodynamic
therapy, the number of pockets ≥7 mm was reduced from 141 to 3
after AB and from 137 to 45 after aPDT. Although both treatments
led to statistically significant clinical improvements, AB showed a
reduction in probing depth and a lower number of pockets ≥7 mm
compared to aPDT. In conclusion, photodynamic therapy associated
with the non-surgical periodontal therapy, despite giving favorable
results, cannot be considered a definitive alternative to the systemic
use of amoxicillin and metronidazole [20]. However, antibiotics
must be administered during or after mechanical therapy since
micro-organisms are particularly protected by biofilm in the
subgingival plaque. With regard to surgical treatment in patients
with aggressive periodontitis, it has been shown that it gives results
comparable to non-surgical treatment provided that correct oral
hygiene is maintained, that a rigorous maintenance program is
followed and that risk factors are kept under editable control [18].
Differential diagnosis
Figure 1:
Periodontal disease is characterized by the imbalance between
pathogens and host defenses leading to an inflammatory reaction
around dental tissues. To diagnose aggressive periodontitis, it
is necessary to investigate the health status of the subject and
exclude the coexistence of systemic diseases. Some disorders
can cause oral lesions clinically similar to those of aggressive
periodontitis, but it is of fundamental importance to distinguish the
various manifestations to make a correct diagnosis and intervene
with the most appropriate treatment. Examples of significant
conditions are AIDS, leukemia, diabetes or rare genetic disorders
such as histiocytosis X and Papillon-Lefevre syndrome. The latter
is a rare autosomal recessive disorder caused by mutations in the
gene that codes for cathepsin C (dipeptidyl-peptidase I inhibitor).
The syndrome is characterized by hyperkeratosis, destructive
periodontitis that occurs from childhood, recurrent piogenic and
systemic skin infections, susceptibility to bacterial infections and
intra-cranial calcifications [21] (Figures 1&2). The prevalence is
estimated to be between 1 / 250,000 and 1 / 1,000,000 subjects and is manifested in all ethnic groups. These dermatological
features appear between the first year of life and 4 years and
are accompanied by intraoral lesions that include gingival
inflammation, mobility of the dental elements, even spontaneous
bleeding and destruction of the periodontium. Patients with this
syndrome show serious signs in the oral cavity until complete
loss of deciduous bone, generating the normal appearance of the
gum. However, with the eruption of permanent teeth, the form
of aggressive periodontitis reappears. Any non-surgical but also
surgical treatment is vain and almost always leads to partial or
complete edentulism in the patient. The treatment is based on
the intake of oral retinoids, which attenuate the palmoplantar
keratoderma and slow down the lysis of the alveolar bone, and the
skin lesions can also be treated with emollients in order to hydrate
the affected area. Furthermore, good oral hygiene control, the use
of mouthwashes and even antibiotics are recommended to slow
the progression of periodontitis. Deciduous teeth or elements with
excessive mobility must be extracted and eventually replaced by
implants when the subject has completed growth.
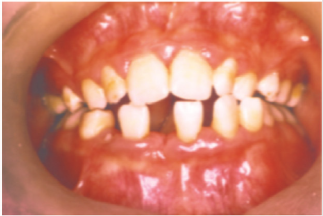
Figure 2:
The identification of the syndrome at an early age is something
multidisciplinary that can improve the patients’ prognosis [22].
Leukemia is a malignant neoplastic disease of white blood cells and
very often strikes in the pediatric age giving oral manifestations
prior to systemic onset [23]. Acute lymphoblastic leukemia (ALL or
ALL), specifically, develops when a cell destined to give rise to cells
of the immune system turns into a tumor and starts to multiply in
an uncontrolled way. Many studies show that acute lymphoblastic
leukemia is the most frequent tumor in children, representing
75% of all newly diagnosed leukemias and 25% of all childhood
malignancies [23]. The typical symptoms that characterize
lymphoblastic leukemia are fatigue, dyspnea, fever, pallor and
weight loss. Patients with this form of leukemia in the oral cavity
have pale mucous membranes and an important gingival bleeding
accompanied by lymphadenopathy in the head and neck region.
It has been shown that sometimes the initial sign of the disease
may correspond to a pericoronitis associated with a prolonged
contraction of the masticatory muscles. Numerous studies have
also reported a greater incidence of abnormalities in the oral cavity
such as the presence of large, irregularly shaped ulcers, halitosis
and a loose mucosa [24].
Acute myeloid leukemia (AML) is a disease that originates
from the bone marrow. The disease is more common in adults
over 60 years and infrequent before the age of 45. Patients with
AML have symptoms related to complications related to anemia,
neutropenia and thrombocytopenia, including weakness and
easy fatigue, infections of varying severity, gingival bleeding,
ecchymosis, epistaxis [25]. The oral examination can show pallor
of the mucosa (Figure 3), ulcerations (Figure 4), spontaneous
bleeding and bleeding (Figure 5) petechiae on gums, palate (Figure
6), tongue or lips, gingival hyperplasia (Figure 7) caused from
leukemic infiltration. Usually the lesions of the oral cavity are the
first manifestations of the disease, in particular, gingival swelling
represents 5% of the early complications [26]. In leukemic patients,
regardless of the form of the disease, oral manifestations occur as
initial evidence or of its recurrence. Symptoms mainly include gum
enlargement and bleeding, oral ulceration, petechiae, mucosal
pallor and oral infections. These lesions may be the result of
direct infiltration of leukemic cells or altered granulocyte function
[26]. With regard to oral hygiene, patients must undergo periodic
checkups and deplaquing or scaling sessions to reduce the level
of inflammation of the mucous membranes, local antiseptics or
antibiotics can be combined with active infections. Chemotherapy,
often used in the treatment of leukemia, also has consequences on
the subject that also affect the oral cavity. Often patients in therapy
are predisposed to the appearance of ulcers, lesions, infections and
are prone to have a partial xerostomia favoring plaque buildup.
Figure 3
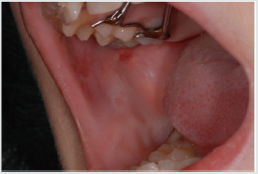
Figure 4:
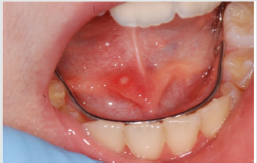
Figure 5:
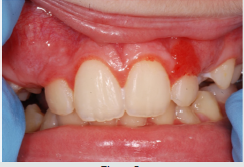
Figure 6:
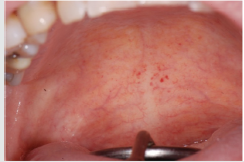
Figure 7:
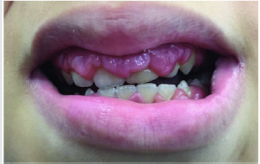
Scurvy is another disease that manifests itself with abundant
gingival bleeding and which can be confused with aggressive
periodontitis. The primary cause of scurvy is the insufficient intake
of vitamin C mainly due to dietary imbalances. Childhood scurvy
generally appears between the sixth and twelfth year of life. The
child is easily irritated without appetite and fatigued. Sufferers of
this disease develop anemia, weakness, fatigue, edema in some
parts of the body, muscle pain in the lower limbs and ulceration of
the gums (Figure 8) [27]. It occurs later as follicular hyperkeratosis
and haemorrhage of the lower limbs, as well as bleeding in other
areas such as the gingiva and joints [28]. If the disease is not treated
it is potentially lethal due to important bleeding that can occur in the
intracranial area or, due to the poor ability of the subject to heal due
to open wound infections. At the oral level, the disease manifests
itself in widespread hypertrophic areas of the violet-colored
mucosa, a tendency for bleeding and the formation of hematomas.
In small subjects, the symptoms that appear first are the pain and
swelling of the joints accompanied by gingival hypertrophy [29].
Figure 8:
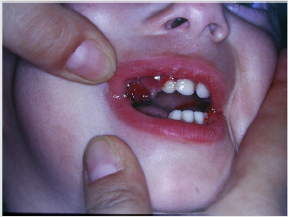
Patients suffering from this pathology are administered
quantities of vitamin C orally or through injections and, in a short
time , all symptoms disappear [28] (Figure 9). Diabetes mellitus is a
disease that can present in the oral cavity as aggressive periodontitis
and be confused with this. It includes a group of chronic metabolic
disorders that turn out to be an altered glucose tolerance or an
altered metabolism of lipids and carbohydrates [30]. It has been
shown by numerous researches that in diabetic children with
poor metabolic control, there is a greater tendency for gingivitis [30].
In fact, the high levels of glucose in the blood cause changes
in microcirculation, promote bacterial proliferation and interact
with the response of the host. Hyperglycemia caused by diabetes
mellitus alters the immune system and the increased availability of
glucose in the oral cavity environment increases the proliferation
of periodontopathic bacteria and causes marked oral inflammation
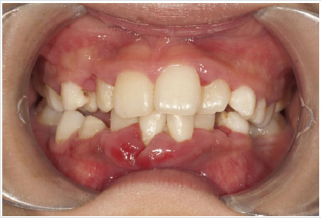
(Figure 10). In patients with diabetes, a microangiopathy occurs
and this change in the periodontium reduces the functions of
the polymorphonuclear cells, the chemotaxis, the adherence, the
phagocytosis, the use of oxygen and the elimination of antigens, thus
favoring the progression of periodontal disease. Hyperglycemia
also reduces the solubility of collagen, reduces the production of
fibroblasts and causes an increase in the levels of pro-inflammatory
mediators responsible for the destruction of connective tissues.
Changes to collagen metabolism result in accelerated degradation
of both non-mineralized connective tissue and mineralized bone.
Figure 9:
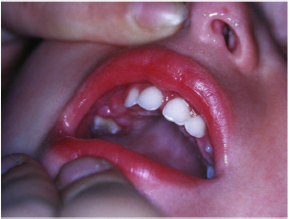
Figure 10:
Even the saliva undergoes both qualitative and quantitative
changes. Often in subjects with diabetes, the salivary flow
is reduced leading to a further development of the bacterial
species [31]. In diabetic patients, periodontal disease develops
at a younger age than the healthy population and periodontal
impairment usually occurs in adolescence but sometimes earlier
in children with diabetes [32]. In the oral cavity, there is therefore
an edematous and very inflamed gingiva, bleeding when the probe
passes and bone resorption can occur especially in cases of poor
metabolic control (Figure 11) [33], but in patients with a good
diet with good glycemic supervision and good oral hygiene, do not show
evident alteration in the mucosa (Figures 12&13). There is
then a relationship between higher levels of plaque and a higher
incidence of gingivitis in children with diabetes, moreover, the
differences in oral microflora and the impact of metabolic control
of diabetes on periodontal health have indicated a higher risk of
periodontitis in children with type 1 diabetes [34]. In conclusion,
when you are confronted with a child who has an oral situation of
persistent inflammation, you need to perform more specific tests
to understand if it is periodontitis as a manifestation of systemic
pathology or aggressive periodontitis itself. The dentist or hygienist
is usually the first to diagnose some diseases due to the involvement
of the periodontium, and it is important to have multidisciplinary
management to try to minimize the physical, psychological and
social effects of the patient at an early age.
Figure 11:
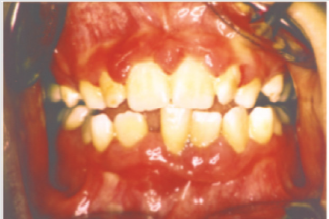
Figure 12:
Figure 13:
Conclusion
In conclusion, the review of the literature shows that
periodontal disease does not only affect adults but, even if less
frequently, it also involves children and adolescents. The forms of
aggressive disease show a family aggregation, cause an important
and rapid destruction even in the absence of local irritative
factors and occur in systemically healthy subjects. It has been
seen that particularly virulent bacteria trigger the process of bone
destruction in the disease, the main micro-organism involved is
Actinomycetemcomitans, which produces powerful leucotoxins that
can also severely damage the subject’s immune system cells. Some
clonal types of the bacterium are very pathogenic, and JP2 is always
isolated from subjects suffering from aggressive periodontitis
indicating that it is an important etiological agent. Regarding the
epidemiological aspects, the statistics show very variable data also
depending on the country, and so far the prevalence of aggressive
periodontitis is not known exactly but it can be said that it occurs
mainly in subjects of African descent and in individuals who are
predisposed from the genetic point of view.
To date there is no specific criterion for defining a high-risk
group for prepubertal pathology, and further research is needed to
identify a robust set of genetic, microbiological risk markers and
host factors that favor a diagnosis of the disease in association with
young people and adolescents. The identification of aggressive
periodontitis can be implemented through periodontal screening
associated with radiographs, and the routine use of BPE can be
helpful for early diagnosis. The treatment consists of the same
methods applied also to adult patients, that is to say a causal therapy,
a mechanic and a pharmacological one, in particular the studies
have shown that antibiotics associated with professional hygiene in
patients with aggressive periodontitis give very satisfactory results.
If, despite careful treatment and hygiene, a child continues to have
periodontal problems, it is necessary to investigate the general
health with more specific examinations. A form of periodontal
injury in a young person can also be a symptom of systemic diseases
that are extraneous to the parent’s awareness and early diagnosis
can become vitally important. Aggressive periodontitis, although
infrequent, is not to be underestimated and it is important to take
children to regular checkups and scaling sessions. Preventing and
diagnosing problems early is the key to success.
Read More Lupine Publishers
Pediatric Dentistry Journal Articles:
https://lupine-publishers-pediatric-dentistry.blogspot.com/